核糖核酸酶A来源于牛胰腺 [RNA酶A],Ribonuclease A from bovine pancreas [RNase A];Type X-A, ≥90% (SDS-PAGE), ≥70 Kunitz units/mg protein
最近浏览商品
-
![显示明细 乙酰胆碱酯酶来源于人 [AChE],Acetylcholinesterase human;recombinant, expressed in HEK 293 cells, lyophilized powder, ≥1,000 units/mg protein (Lowry) 图片 乙酰胆碱酯酶来源于人 [AChE],Acetylcholinesterase human;recombinant, expressed in HEK 293 cells, lyophilized powder, ≥1,000 units/mg protein (Lowry)](https://www.bszh.cn/images/thumbs/0004784_-acheacetylcholinesterase-humanrecombinant-expressed-in-hek-293-cells-lyophilized-powder-1000-unitsm_32.jpeg) 乙酰胆碱酯酶来源于人 [AChE],Acetylcholinesterase human;recombinant, expressed in HEK 293 cells, lyophilized powder, ≥1,000 units/mg protein (Lowry)
乙酰胆碱酯酶来源于人 [AChE],Acetylcholinesterase human;recombinant, expressed in HEK 293 cells, lyophilized powder, ≥1,000 units/mg protein (Lowry) -
![显示明细 人脱铁转铁蛋白 [人转铁蛋白],apo-Transferrin human;powder, BioReagent, suitable for cell culture, ≥98% (agarose gel electrophoresis) 图片 人脱铁转铁蛋白 [人转铁蛋白],apo-Transferrin human;powder, BioReagent, suitable for cell culture, ≥98% (agarose gel electrophoresis)](https://www.bszh.cn/images/thumbs/0015699_-apo-transferrin-humanpowder-bioreagent-suitable-for-cell-culture-98-agarose-gel-electrophoresis_32.webp) 人脱铁转铁蛋白 [人转铁蛋白],apo-Transferrin human;powder, BioReagent, suitable for cell culture, ≥98% (agarose gel electrophoresis)
人脱铁转铁蛋白 [人转铁蛋白],apo-Transferrin human;powder, BioReagent, suitable for cell culture, ≥98% (agarose gel electrophoresis) -
 过氧化氢酶来源于人类红细胞,Catalase from human erythrocytes;≥90% (SDS-PAGE), buffered aqueous solution, ≥30,000 units/mg protein
过氧化氢酶来源于人类红细胞,Catalase from human erythrocytes;≥90% (SDS-PAGE), buffered aqueous solution, ≥30,000 units/mg protein -
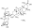 甘草酸铵盐,Glycyrrhizic acid ammonium salt;analytical standard, suitable for HPLC, ≥90.0% (HPLC)
甘草酸铵盐,Glycyrrhizic acid ammonium salt;analytical standard, suitable for HPLC, ≥90.0% (HPLC) -
![显示明细 核糖核酸酶A来源于牛胰腺 [RNA酶A],Ribonuclease A from bovine pancreas [RNase A];Type XII-A, ≥90% (SDS-PAGE), 75-125 Kunitz units/mg protein 图片 核糖核酸酶A来源于牛胰腺 [RNA酶A],Ribonuclease A from bovine pancreas [RNase A];Type XII-A, ≥90% (SDS-PAGE), 75-125 Kunitz units/mg protein](https://www.bszh.cn/images/thumbs/0004840_a-rnaaribonuclease-a-from-bovine-pancreas-rnase-atype-xii-a-90-sds-page-75-125-kunitz-unitsmg-protei_32.jpeg) 核糖核酸酶A来源于牛胰腺 [RNA酶A],Ribonuclease A from bovine pancreas [RNase A];Type XII-A, ≥90% (SDS-PAGE), 75-125 Kunitz units/mg protein
核糖核酸酶A来源于牛胰腺 [RNA酶A],Ribonuclease A from bovine pancreas [RNase A];Type XII-A, ≥90% (SDS-PAGE), 75-125 Kunitz units/mg protein -
![显示明细 异柠檬酸脱氢酶1来源于人,Isocitrate Dehydrogenase 1 (NADP+) human [IDH1];recombinant, expressed in E. coli, lyophilized powder, ≥80 units/mg protein 图片 异柠檬酸脱氢酶1来源于人,Isocitrate Dehydrogenase 1 (NADP+) human [IDH1];recombinant, expressed in E. coli, lyophilized powder, ≥80 units/mg protein](https://www.bszh.cn/images/thumbs/0016282_1isocitrate-dehydrogenase-1-nadp-human-idh1recombinant-expressed-in-e-coli-lyophilized-powder-80-uni_32.jpeg) 异柠檬酸脱氢酶1来源于人,Isocitrate Dehydrogenase 1 (NADP+) human [IDH1];recombinant, expressed in E. coli, lyophilized powder, ≥80 units/mg protein
异柠檬酸脱氢酶1来源于人,Isocitrate Dehydrogenase 1 (NADP+) human [IDH1];recombinant, expressed in E. coli, lyophilized powder, ≥80 units/mg protein

